
Simple, Efficient, Multi-Night Home Sleep Testing and Diagnosis
Accelerate the identification, diagnosis, and treatment of sleep disordered breathing with accurate, affordable, AI-driven analysis of photoplethysmogram (PPG) signals captured by pulse oximeters. EnsoHST™ measures respiratory activity based on changes in blood volume and peripheral arterial tone.
Expand Patient Access and Advance Home Sleep Testing

-
Up to 7 nights of testing per patient
-
Affordable, comfortable, non-proprietary devices
-
Reusable or a single-patient disposable device
-
Dynamic reports for all devices in one platform
-
Accurate, unbiased, and clinically effective
Enhance Sleep Disorder Diagnosis and Long-Term Monitoring
-
FDA-cleared AI analysis powered by deep learning models
-
PPG-based studies validated against PSGs
-
Detects sleep disordered breathing events
-
Identifies sleep stages (REM, Deep Sleep, Light Sleep)
-
Displays PPG, HRV, pulse, snore, acoustic flow, SpO2, actigraphy, position, and surrogate effort data
-
Provides an easy-to-read, editable, and exportable sleep study for physician review
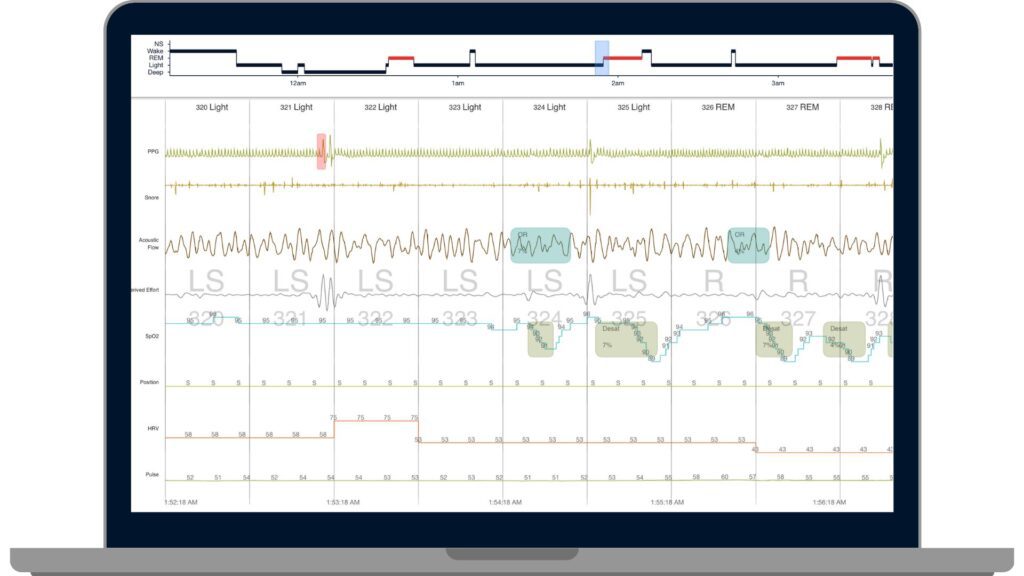
Access, review, and edit all AI-scored HSAT, PSG, and PPG-based sleep studies in one platform
Case Study: Reduce Costs, Expedite Outcomes with EnsoHST
In just 3 months, Chicago ENT/Chicago Sleep Center (CENT/CSC) transitioned more than 75% of their home sleep testing volume from flow-based devices to PPG-based devices, corresponding to a 92.5% reduction in cost per new HSAT device.
Access the Case Study to explore CENT/CSC’s rapid and efficient adoption.
"We went from a $2,000 piece of hardware to a $150 piece of hardware, with no disposables. Those economics make EnsoData's solution very attractive."
Scott Rice, Director of Ancillary Services

Celeste+ is a mobile app (Android and iOS) used to seamlessly collect and transfer data from pulse oximeters to EnsoHST.
EnsoHST Clinical Performance
These key metrics demonstrate the clinical impact of EnsoHST

Multi-Night Testing Addresses Inter-Night Variability
Relying on a single night of testing can lead to missed diagnoses in more than 1 in 10 patients.1 More than 1 in 4 patients1 may experience a jump in OSA severity by at least one category on subsequent nights. This can prevent patients from receiving the diagnosis and treatment they need to improve their overall health.
“The high rate of missed diagnosis due to single-night testing gives the sleep medicine community the opportunity to reexamine current testing methods and explore the benefits of multi-night testing. In terms of clinical effectiveness and the future of sleep medicine diagnostics, it may well be more productive to monitor one polysomnographic component for 20 nights rather than monitoring 20 components for one night.”
Nathaniel F. Watson, MD, MSc, Chief Medical Officer, EnsoData, AASM Past-President

Long-Term Therapy Monitoring and Titration
Confidently titrate and monitor patient success on OSA therapies such as CPAP, HGNS, oral appliances, and GLP-1s with long-term nightly measurement of AHI using affordable, patient-friendly devices at home.
Schedule a Demo
Explore EnsoHST for simple, efficient, multi-night home sleep testing
Complete our form and we’ll reach out to schedule a demo and answer your questions about EnsoHST.
We’d love to help you reach more patients and accelerate the identification, diagnosis, and treatment of sleep-disordered breathing with accurate, affordable, multi-night home sleep testing using comfortable devices.
